Ion exchange usually describes a process of purification of aqueous solutions using solid polymeric ion-exchange resin. Activated carbon is produced specifically so as to achieve a very big internal surface (between 500 - 1500 m 2 /g). Abstract. Nitrate and phosphate. There was an increase in ion exchange capacity with increase in adsorbent dosage due to the availability of more adsorption sites on the adsorbent for metal ion adsorption. tertiary/advanced wastewater treatment methods such as ion exchange, precipitation, membrane separation, electrolysis and adsorption_ can be used to remove these recalcitrant wastes. Filtration is a physical separation process that separates solid matter and fluid from a mixture using a filter medium that has a complex structure through which only the fluid can pass. However, this limit M-alk. if calcium and magnesium are the predominant cations adsorbed on the soil 57.14). Water treatment is any process that improves the quality of water to make it appropriate for a specific end-use. Manuscript Generator Search Engine. The development of cost-effective and stable materials and methods for providing the fresh water in adequate amounts is the need of the water industry. Adsorption is a surface phenomenon, while absorption involves Then, 15 mL 0.1 M KMnO 4 aqueous solution was slowly added into the above solution. Oversize particles may form a filter cake on top of the filter Ion exchange process uses an ion exchange membrane which potentially eliminates colloidal and soluble ionic contaminants Advances in water treatment by adsorption technology. Hexavalent chromium was found in drinking water in the southern California town of Hinkley and was brought to popular attention by the involvement of Erin Brockovich and Attorney Edward Masry.The source of contamination was from the evaporating ponds of a PG&E (Pacific Gas and Electric) natural gas pipeline compressor station about 2 miles southeast of Hinkley. The process of adsorption treatment of effluents from ammonium ions is considered as an integrated two-stage process consisting of the stage of adsorption of contaminants by natural sorbents in the apparatus with a stirrer and the liquid separation stage and solid phases.  Mechanisms of the process are explained and a kinetic process is formulated. (2011) and Rao et al. Ion Exchange as a Sorption Process Since ion exchange occurs between a solution and the internal surface of a solid it can be viewed as a special type of sorption process There are many similarities between adsorption and ion exchange. This is because sodium when present in the soil in exchangeable form replaces calcium and magnesium adsorbed on the soil clays and causes dispersion of soil particles (i.e. Centrifuging separates the PVP coated nanoparticles which are then transferred to a solution of ethanol to be centrifuged further and placed in a solution of ammonia, ethanol and Si(OEt 4) Ion exchange is a reversible interchange of one kind of ion present in an insoluble solid with another of like charge present in a solution surrounding the solid with the reaction being used especially for softening or making water demineralised, the purification of chemicals and separation of substances.. Ion exchange usually describes a process of purification of
Mechanisms of the process are explained and a kinetic process is formulated. (2011) and Rao et al. Ion Exchange as a Sorption Process Since ion exchange occurs between a solution and the internal surface of a solid it can be viewed as a special type of sorption process There are many similarities between adsorption and ion exchange. This is because sodium when present in the soil in exchangeable form replaces calcium and magnesium adsorbed on the soil clays and causes dispersion of soil particles (i.e. Centrifuging separates the PVP coated nanoparticles which are then transferred to a solution of ethanol to be centrifuged further and placed in a solution of ammonia, ethanol and Si(OEt 4) Ion exchange is a reversible interchange of one kind of ion present in an insoluble solid with another of like charge present in a solution surrounding the solid with the reaction being used especially for softening or making water demineralised, the purification of chemicals and separation of substances.. Ion exchange usually describes a process of purification of  Ion-Exchange - A chemical process involving the reversible interchange of ions between a solution and a particular solid material (ionexchanger), such as an ion-exchange resin consisting of matrix of insoluble material interspersed with fixed ions of opposite charge. New generation adsorbents for water treatment. Our customers include drinking water, industrial process, mining, dewatering, construction, chemical, remediation, & general tertiary wastewater treatment. Our customers include drinking water, industrial process, mining, dewatering, construction, chemical, remediation, & general tertiary wastewater treatment. So far, a number of efficient methods have been developed for heavy metal removal. Youve supercharged your research process with ACS and Mendeley! The Bodyguard Plus is a great budget friendly whole-house municipal filtration system, offered by popular water treatment brand, US Water Systems. In the ion exchange process, metal ions must migrate through the pores of the zeolite mass and the channels of the lattice to be replaced by exchangeable cations mainly calcium and sodium. Emerging pollutants removal through advanced drinking water treatment: A review on processes and environmental performances assessment. The ion exchange resin treatment step can be used to recover acetic acid. 3.6. Continue. Nitrite 100% Nitrate 98% Colour 98% Turbidity 98% COD 74% COD 83.3% Kefir grains/Ag-doped TiO 2 photocatalytic Biological pre-treatment was done in 250 mL beakers containing 50 mL of leachate inoculated with Kefir grains. waste water for advanced treatment). SAMCO designs, manufactures, and services custom integrated water, wastewater, and process purification and separation equipment systems. However, when the excessive adsorbent is coated on the electrode could significantly fortifying the ion transfer resistance, so that the optimum amount of adsorbent is 5.87 mg. In this method, polyvinylpyrrolidone (PVP) is dissolved in water by sonication and mixed with silver colloid particles. Major Aluminum Manufacturer Treats Chemical Bath With SAMCO Ion Exchange, Avoids Need to Discharge Waste Leveraging SAMCO Ion Exchange Technology for Wastewater Treatment Read Project Brief. Claudia Cobzaru, Vassilis Inglezakis, in Progress in Filtration and Separation, 2015.
Ion-Exchange - A chemical process involving the reversible interchange of ions between a solution and a particular solid material (ionexchanger), such as an ion-exchange resin consisting of matrix of insoluble material interspersed with fixed ions of opposite charge. New generation adsorbents for water treatment. Our customers include drinking water, industrial process, mining, dewatering, construction, chemical, remediation, & general tertiary wastewater treatment. Our customers include drinking water, industrial process, mining, dewatering, construction, chemical, remediation, & general tertiary wastewater treatment. So far, a number of efficient methods have been developed for heavy metal removal. Youve supercharged your research process with ACS and Mendeley! The Bodyguard Plus is a great budget friendly whole-house municipal filtration system, offered by popular water treatment brand, US Water Systems. In the ion exchange process, metal ions must migrate through the pores of the zeolite mass and the channels of the lattice to be replaced by exchangeable cations mainly calcium and sodium. Emerging pollutants removal through advanced drinking water treatment: A review on processes and environmental performances assessment. The ion exchange resin treatment step can be used to recover acetic acid. 3.6. Continue. Nitrite 100% Nitrate 98% Colour 98% Turbidity 98% COD 74% COD 83.3% Kefir grains/Ag-doped TiO 2 photocatalytic Biological pre-treatment was done in 250 mL beakers containing 50 mL of leachate inoculated with Kefir grains. waste water for advanced treatment). SAMCO designs, manufactures, and services custom integrated water, wastewater, and process purification and separation equipment systems. However, when the excessive adsorbent is coated on the electrode could significantly fortifying the ion transfer resistance, so that the optimum amount of adsorbent is 5.87 mg. In this method, polyvinylpyrrolidone (PVP) is dissolved in water by sonication and mixed with silver colloid particles. Major Aluminum Manufacturer Treats Chemical Bath With SAMCO Ion Exchange, Avoids Need to Discharge Waste Leveraging SAMCO Ion Exchange Technology for Wastewater Treatment Read Project Brief. Claudia Cobzaru, Vassilis Inglezakis, in Progress in Filtration and Separation, 2015.  tertiary/advanced wastewater treatment methods such as ion exchange, precipitation, membrane separation, electrolysis and adsorption_ can be used to remove these recalcitrant wastes. Solid particles that cannot pass through the filter medium are described as oversize and the fluid that passes through is called the filtrate. When water is too hard, it is difficult to use to clean and often leaves a grey residue. And if amounts of reagent are precisely adjusted, the waters alkalinity will be reduced to the theoretical solubility applicable to the CaCO 3 + Mg (OH) 2 system which ranges from 2 to 3F under normal temperature and concentration conditions.. There are a lot of different types of desorption, depending on the mechanism that separates the adsorbate from the substrate; therefore there (2011) and Rao et al. Water balance for a fermentation process manufacturing plant producing penicillin (ratio of consumption of process water to total water = 0.08). In the first part of the chapter, the fundamentals of ion exchange and adsorption processes are explained, with the goal of demonstrating how these principles influence process design for inorganic contaminant removal. In low-concentration brackish water, the non-permselective ion-exchange regime dominates only at the lowest state of charge (Fig. Adsorption Adsorption is a mass transfer process in which substances present in the liquid phase are adsorbed or accumulated on a solid phase and are removed from the liquid. Ion exchange. Ion exchange is a technique that involves exchanging charged inorganic pollutants such as arsenic, chromium, nitrate, radium, uranium, and excess fluoride for innocuous charged ions on a resins surface. (C) We demonstrate the use of these adsorptive membranes in an electrodialysis-based process for the selective capture of target cations (right-hand side) from water and simultaneous desalination.Water splitting Sodium hazard of irrigation water High sodium ions in water affects the permeability of soil and causes infiltration problems. Keep in mind that resin materials can only be charged for a specific period of time. Along with absorption and adsorption, ion exchange is a form of sorption. The choice of product for a specific application will vary due to differing impurities and proprietary process conditions. Ion exchange resins consist of polymer beads that are chemically engineered to suit specific ion exchange reactions. Calcium and magnesium are common ions that lead to water hardness. Ion Exchange India Ltd | 31,115 followers on LinkedIn. The exchange occurs between the ions of insoluble exchange material (ion-exchange materials) and the ions of different species in solution (i.e. Filtration is a physical separation process that separates solid matter and fluid from a mixture using a filter medium that has a complex structure through which only the fluid can pass. When water is too hard, it is difficult to use to clean and often leaves a grey residue. Historical Perspectives Ion Exchange Natural phenomena that occurs in soil, minerals and tissues of plants and animals Thompson and Way (1850) first described process in soils Eichorn (1858) demonstrated that process in reversible Gans (1905) developed first practical ion exchange process for softening water using sodium aluminates The rapidly increasing population, depleting water resources, and climate change resulting in prolonged droughts and floods have rendered drinking water a competitive resource in many parts of the world. The initial pH of the solution affected the adsorption effect of biochar on heavy metals, and the adsorption effect of different pH on mercury ion was investigated in Fig. Desorption is the physical process where a previously adsorbed substance is released from a surface. Municipal Water Treatment; Fixed Bed; internal pore structures are created by imparting unique adsorption properties specific to each product type. Activated carbon adsorption. An ion exchange process, similar to the reverse osmosis process, can be used to soften the water. Ion Exchange. Emerging pollutants removal through advanced drinking water treatment: A review on processes and environmental performances assessment. Basic concept of adsorption, its types (i.e., chemisorption and physisorption) and its mechanism, adsorbents and adsorbates were included. An ion exchange process, similar to the reverse osmosis process, can be used to soften the water. ), Water Treatment (pp. (A and B) Tunable composite membranes were prepared by embedding PAFs with selective ion binding sites into cation exchange polymer matrices. Wastewater Treatment by Ion Exchange 691. The adsorption of polyelectrolytes on to pure crystals is different from that on to most other surfaces, particularly at high pH, where significant amounts of polymer can be adsorbed. Water balance for a fermentation process manufacturing plant producing penicillin (ratio of consumption of process water to total water = 0.08). US government agency endorses tools to keep the Internet safe from quantum computers capable of cracking conventional encryption keys. (This is why clothing washed in hard water often retains a dingy tint.) Adsorption is a process where a solid is used for removing a soluble substance from the water. This is likely to be a result of two factors, the first of which is that, although crystals may have an overall negative Advanced oxidation process/Adsorption (ion-exchange) Scandelai et al. The ion exchange process will take place between a liquid and a solid. Active stirring ensures the PVP has adsorbed to the nanoparticle surface. However, when the excessive adsorbent is coated on the electrode could significantly fortifying the ion transfer resistance, so that the optimum amount of adsorbent is 5.87 mg. Sentence Examples. Key Features: In the first section of the present chapter the kinetics and This happens when a molecule gains enough energy to overcome the activation barrier of the bounding energy that keeps it in the surface.. Lecture 45: Tertiary Treatment: Adsorption and Ion Exchange There are a lot of different types of desorption, depending on the mechanism that separates the adsorbate from the substrate; therefore there Ion Exchange is globally recognized as a premier company in Water & Environment management with a legacy spanning over | At Ion Exchange India, we bring you total environment solutions - water treatment, liquid waste treatment & recycle, air pollution control, solid & hazardous waste management and generation Adsorption is the adhesion of atoms, ions or molecules from a gas, liquid or dissolved solid to a surface. Complete study of Ion Exchange; Municipal Water Treatment. A locked padlock) or https:// means youve safely connected to the .gov website. 1a).The first step constructed a chitosan hybrid hydrogel with dispersed thiosemicarbazide, Fe(acac) 3, MgCl 2, and KOH.Through the reaction of MgCl 2 with KOH, the composite templates of Mg(OH) 2 and KCl were in-situ prepared. While the liquid is always water, the solid can be either zeolite or a resin material. The Bodyguard Plus is a great budget friendly whole-house municipal filtration system, offered by popular water treatment brand, US Water Systems. Continue. if calcium and magnesium are the predominant cations adsorbed on the soil waste water for advanced treatment). Present paper involved the review of fixed-bed column studies for removal of various contaminants from synthetic wastewater. Perchlorate contamination in food, water, and other parts of the environment has been studied in the U.S. because of harmful The ion-exchange process is a natural phenomenon and mankind has been using this technique since the early days of civilisation. They are mainly used as oxidizers for pyrotechnic devices and to control static electricity in food packaging. Nature Protocols, 1(6), pp. (2011) reviewed various The authors then present the Water Quality Conversion Matrix or Water Quality Treatment Matrix. H 2 O was added to 15 mL deionized water and stirred it until a clear solution was obtained. Adsorption and ion exchange are two sorption mechanisms by which PFAS can be removed from water. Fenglian et al. Natural resources (6) In this method, polyvinylpyrrolidone (PVP) is dissolved in water by sonication and mixed with silver colloid particles. We specialize in the design, development, fabrication, & supply of water treatment solutions that remove a wide range of contaminants from water. Ion Exchange. The development of cost-effective and stable materials and methods for providing the fresh water in adequate amounts is the need of the water industry. The FeNSC-nFe catalysts were synthesized using a multi-step pyrolysis process (Fig. The functions of the activated carbon adsorption step include: (1) remove lignin and other relatively high molecular weight organics; (2) facilitate the subsequent operations, such as membrane filtration and ion exchange resin treatment. Ion exchange. This is likely to be a result of two factors, the first of which is that, although crystals may have an overall negative Therefore, the aim of this Special Issue, entitled Wastewater Treatment by Adsorption and/or Ion-Exchange Processes for Resource Recovery, is to promote these two processes as innovative and environmentally friendly alternatives for the recovery of secondary raw materials from by-products or waste streams. (Eds. Traditional The process of ion exchange is one of the most utilized techniques in water and wastewater treatment as well as in separation processes as chemical synthesis, medical research, food processing, mining, agriculture, etc. In this process active carbon is the solid. Ion exchange is a water treatment process commonly used for water softening or demineralization, but it also is used to remove other substances from the water in processes such as dealkalization, deionization, denitrification, and disinfection. Basic concept of adsorption, its types (i.e., chemisorption and physisorption) and its mechanism, adsorbents and adsorbates were included. Youve supercharged your research process with ACS and Mendeley! Ion exchange is a water treatment process commonly used for water softening or demineralization, but it also is used to remove other substances from the water in processes such as dealkalization, deionization, denitrification, and disinfection. There was an increase in ion exchange capacity with increase in adsorbent dosage due to the availability of more adsorption sites on the adsorbent for metal ion adsorption. The adsorption capacity of biochar was weak at pH = 2, and the adsorption capacity of KRSB and RSB was only 108.3 mg/g and 9.65 mg/g, which was due to the adsorption environment being more acidic. Those technologies include activated carbon adsorption, ion exchange resins, and high-pressure membranes. Another application for ion exchange in domestic water treatment is the removal of nitrate and natural organic matter. Present paper involved the review of fixed-bed column studies for removal of various contaminants from synthetic wastewater. Fenglian et al. Oversize particles may form a filter cake on top of the filter Complete study of The exchange occurs between the ions of insoluble exchange material (ion-exchange materials) and the ions of different species in solution (i.e. The uptake of heavy metal is attributed to several mechanisms in the ion-exchange process and the adsorption process. In this study, novel water treatment residuals nanoparticles (nWTRs) were prepared using high energy ball milling and used for efficient removal of Cd(II) in single-and multi-ion systems. Advanced oxidation process/Adsorption (ion-exchange) Scandelai et al. 57.14). The initial pH of the solution affected the adsorption effect of biochar on heavy metals, and the adsorption effect of different pH on mercury ion was investigated in Fig. This is because sodium when present in the soil in exchangeable form replaces calcium and magnesium adsorbed on the soil clays and causes dispersion of soil particles (i.e. The adsorption capacity of biochar was weak at pH = 2, and the adsorption capacity of KRSB and RSB was only 108.3 mg/g and 9.65 mg/g, which was due to the adsorption environment being more acidic. The rapidly increasing population, depleting water resources, and climate change resulting in prolonged droughts and floods have rendered drinking water a competitive resource in many parts of the world. In the second part, ion exchange and adsorption processes that have been proven effective at bench, pilot, and full scale are described for the This system is available in 3 flow rates: 10, 15 and 20 GPM, and can reduce or remove a combination of the most common drinking water contaminants. This happens when a molecule gains enough energy to overcome the activation barrier of the bounding energy that keeps it in the surface.. Treatment of Tannery Wastewater by Various Oxidation and Combined Processes There are various technologies used for removing arsenic from drinking water. organic acids, ammonium, It can be deduced from the above research that pore filling and intra-particle diffusion play an important role in the adsorption process of many ECs. Documents; Authors; Tables; Documents: Advanced Search Include Citations Authors: Advanced Search Include Citations Tables: Pretreatment of tannery wastewaters by an ion exchange process for Cr(III) removal and recovery.
tertiary/advanced wastewater treatment methods such as ion exchange, precipitation, membrane separation, electrolysis and adsorption_ can be used to remove these recalcitrant wastes. Solid particles that cannot pass through the filter medium are described as oversize and the fluid that passes through is called the filtrate. When water is too hard, it is difficult to use to clean and often leaves a grey residue. And if amounts of reagent are precisely adjusted, the waters alkalinity will be reduced to the theoretical solubility applicable to the CaCO 3 + Mg (OH) 2 system which ranges from 2 to 3F under normal temperature and concentration conditions.. There are a lot of different types of desorption, depending on the mechanism that separates the adsorbate from the substrate; therefore there (2011) and Rao et al. Water balance for a fermentation process manufacturing plant producing penicillin (ratio of consumption of process water to total water = 0.08). In the first part of the chapter, the fundamentals of ion exchange and adsorption processes are explained, with the goal of demonstrating how these principles influence process design for inorganic contaminant removal. In low-concentration brackish water, the non-permselective ion-exchange regime dominates only at the lowest state of charge (Fig. Adsorption Adsorption is a mass transfer process in which substances present in the liquid phase are adsorbed or accumulated on a solid phase and are removed from the liquid. Ion exchange. Ion exchange is a technique that involves exchanging charged inorganic pollutants such as arsenic, chromium, nitrate, radium, uranium, and excess fluoride for innocuous charged ions on a resins surface. (C) We demonstrate the use of these adsorptive membranes in an electrodialysis-based process for the selective capture of target cations (right-hand side) from water and simultaneous desalination.Water splitting Sodium hazard of irrigation water High sodium ions in water affects the permeability of soil and causes infiltration problems. Keep in mind that resin materials can only be charged for a specific period of time. Along with absorption and adsorption, ion exchange is a form of sorption. The choice of product for a specific application will vary due to differing impurities and proprietary process conditions. Ion exchange resins consist of polymer beads that are chemically engineered to suit specific ion exchange reactions. Calcium and magnesium are common ions that lead to water hardness. Ion Exchange India Ltd | 31,115 followers on LinkedIn. The exchange occurs between the ions of insoluble exchange material (ion-exchange materials) and the ions of different species in solution (i.e. Filtration is a physical separation process that separates solid matter and fluid from a mixture using a filter medium that has a complex structure through which only the fluid can pass. When water is too hard, it is difficult to use to clean and often leaves a grey residue. Historical Perspectives Ion Exchange Natural phenomena that occurs in soil, minerals and tissues of plants and animals Thompson and Way (1850) first described process in soils Eichorn (1858) demonstrated that process in reversible Gans (1905) developed first practical ion exchange process for softening water using sodium aluminates The rapidly increasing population, depleting water resources, and climate change resulting in prolonged droughts and floods have rendered drinking water a competitive resource in many parts of the world. The initial pH of the solution affected the adsorption effect of biochar on heavy metals, and the adsorption effect of different pH on mercury ion was investigated in Fig. Desorption is the physical process where a previously adsorbed substance is released from a surface. Municipal Water Treatment; Fixed Bed; internal pore structures are created by imparting unique adsorption properties specific to each product type. Activated carbon adsorption. An ion exchange process, similar to the reverse osmosis process, can be used to soften the water. Ion Exchange. Emerging pollutants removal through advanced drinking water treatment: A review on processes and environmental performances assessment. Basic concept of adsorption, its types (i.e., chemisorption and physisorption) and its mechanism, adsorbents and adsorbates were included. An ion exchange process, similar to the reverse osmosis process, can be used to soften the water. ), Water Treatment (pp. (A and B) Tunable composite membranes were prepared by embedding PAFs with selective ion binding sites into cation exchange polymer matrices. Wastewater Treatment by Ion Exchange 691. The adsorption of polyelectrolytes on to pure crystals is different from that on to most other surfaces, particularly at high pH, where significant amounts of polymer can be adsorbed. Water balance for a fermentation process manufacturing plant producing penicillin (ratio of consumption of process water to total water = 0.08). US government agency endorses tools to keep the Internet safe from quantum computers capable of cracking conventional encryption keys. (This is why clothing washed in hard water often retains a dingy tint.) Adsorption is a process where a solid is used for removing a soluble substance from the water. This is likely to be a result of two factors, the first of which is that, although crystals may have an overall negative Advanced oxidation process/Adsorption (ion-exchange) Scandelai et al. The ion exchange process will take place between a liquid and a solid. Active stirring ensures the PVP has adsorbed to the nanoparticle surface. However, when the excessive adsorbent is coated on the electrode could significantly fortifying the ion transfer resistance, so that the optimum amount of adsorbent is 5.87 mg. Sentence Examples. Key Features: In the first section of the present chapter the kinetics and This happens when a molecule gains enough energy to overcome the activation barrier of the bounding energy that keeps it in the surface.. Lecture 45: Tertiary Treatment: Adsorption and Ion Exchange There are a lot of different types of desorption, depending on the mechanism that separates the adsorbate from the substrate; therefore there Ion Exchange is globally recognized as a premier company in Water & Environment management with a legacy spanning over | At Ion Exchange India, we bring you total environment solutions - water treatment, liquid waste treatment & recycle, air pollution control, solid & hazardous waste management and generation Adsorption is the adhesion of atoms, ions or molecules from a gas, liquid or dissolved solid to a surface. Complete study of Ion Exchange; Municipal Water Treatment. A locked padlock) or https:// means youve safely connected to the .gov website. 1a).The first step constructed a chitosan hybrid hydrogel with dispersed thiosemicarbazide, Fe(acac) 3, MgCl 2, and KOH.Through the reaction of MgCl 2 with KOH, the composite templates of Mg(OH) 2 and KCl were in-situ prepared. While the liquid is always water, the solid can be either zeolite or a resin material. The Bodyguard Plus is a great budget friendly whole-house municipal filtration system, offered by popular water treatment brand, US Water Systems. Continue. if calcium and magnesium are the predominant cations adsorbed on the soil waste water for advanced treatment). Present paper involved the review of fixed-bed column studies for removal of various contaminants from synthetic wastewater. Perchlorate contamination in food, water, and other parts of the environment has been studied in the U.S. because of harmful The ion-exchange process is a natural phenomenon and mankind has been using this technique since the early days of civilisation. They are mainly used as oxidizers for pyrotechnic devices and to control static electricity in food packaging. Nature Protocols, 1(6), pp. (2011) reviewed various The authors then present the Water Quality Conversion Matrix or Water Quality Treatment Matrix. H 2 O was added to 15 mL deionized water and stirred it until a clear solution was obtained. Adsorption and ion exchange are two sorption mechanisms by which PFAS can be removed from water. Fenglian et al. Natural resources (6) In this method, polyvinylpyrrolidone (PVP) is dissolved in water by sonication and mixed with silver colloid particles. We specialize in the design, development, fabrication, & supply of water treatment solutions that remove a wide range of contaminants from water. Ion Exchange. The development of cost-effective and stable materials and methods for providing the fresh water in adequate amounts is the need of the water industry. The FeNSC-nFe catalysts were synthesized using a multi-step pyrolysis process (Fig. The functions of the activated carbon adsorption step include: (1) remove lignin and other relatively high molecular weight organics; (2) facilitate the subsequent operations, such as membrane filtration and ion exchange resin treatment. Ion exchange. This is likely to be a result of two factors, the first of which is that, although crystals may have an overall negative Therefore, the aim of this Special Issue, entitled Wastewater Treatment by Adsorption and/or Ion-Exchange Processes for Resource Recovery, is to promote these two processes as innovative and environmentally friendly alternatives for the recovery of secondary raw materials from by-products or waste streams. (Eds. Traditional The process of ion exchange is one of the most utilized techniques in water and wastewater treatment as well as in separation processes as chemical synthesis, medical research, food processing, mining, agriculture, etc. In this process active carbon is the solid. Ion exchange is a water treatment process commonly used for water softening or demineralization, but it also is used to remove other substances from the water in processes such as dealkalization, deionization, denitrification, and disinfection. Basic concept of adsorption, its types (i.e., chemisorption and physisorption) and its mechanism, adsorbents and adsorbates were included. Youve supercharged your research process with ACS and Mendeley! Ion exchange is a water treatment process commonly used for water softening or demineralization, but it also is used to remove other substances from the water in processes such as dealkalization, deionization, denitrification, and disinfection. There was an increase in ion exchange capacity with increase in adsorbent dosage due to the availability of more adsorption sites on the adsorbent for metal ion adsorption. The adsorption capacity of biochar was weak at pH = 2, and the adsorption capacity of KRSB and RSB was only 108.3 mg/g and 9.65 mg/g, which was due to the adsorption environment being more acidic. Those technologies include activated carbon adsorption, ion exchange resins, and high-pressure membranes. Another application for ion exchange in domestic water treatment is the removal of nitrate and natural organic matter. Present paper involved the review of fixed-bed column studies for removal of various contaminants from synthetic wastewater. Fenglian et al. Oversize particles may form a filter cake on top of the filter Complete study of The exchange occurs between the ions of insoluble exchange material (ion-exchange materials) and the ions of different species in solution (i.e. The uptake of heavy metal is attributed to several mechanisms in the ion-exchange process and the adsorption process. In this study, novel water treatment residuals nanoparticles (nWTRs) were prepared using high energy ball milling and used for efficient removal of Cd(II) in single-and multi-ion systems. Advanced oxidation process/Adsorption (ion-exchange) Scandelai et al. 57.14). The initial pH of the solution affected the adsorption effect of biochar on heavy metals, and the adsorption effect of different pH on mercury ion was investigated in Fig. This is because sodium when present in the soil in exchangeable form replaces calcium and magnesium adsorbed on the soil clays and causes dispersion of soil particles (i.e. The adsorption capacity of biochar was weak at pH = 2, and the adsorption capacity of KRSB and RSB was only 108.3 mg/g and 9.65 mg/g, which was due to the adsorption environment being more acidic. The rapidly increasing population, depleting water resources, and climate change resulting in prolonged droughts and floods have rendered drinking water a competitive resource in many parts of the world. In the second part, ion exchange and adsorption processes that have been proven effective at bench, pilot, and full scale are described for the This system is available in 3 flow rates: 10, 15 and 20 GPM, and can reduce or remove a combination of the most common drinking water contaminants. This happens when a molecule gains enough energy to overcome the activation barrier of the bounding energy that keeps it in the surface.. Treatment of Tannery Wastewater by Various Oxidation and Combined Processes There are various technologies used for removing arsenic from drinking water. organic acids, ammonium, It can be deduced from the above research that pore filling and intra-particle diffusion play an important role in the adsorption process of many ECs. Documents; Authors; Tables; Documents: Advanced Search Include Citations Authors: Advanced Search Include Citations Tables: Pretreatment of tannery wastewaters by an ion exchange process for Cr(III) removal and recovery.  Adsorption is a physical mass transfer process that uses Van der Waals and/or other weak ionic forces to bind the entire PFAS molecule to the surface areas of the adsorptive media. Adsorption is a physical mass transfer process that uses Van der Waals and/or other weak ionic forces to bind the entire PFAS molecule to the surface areas of the adsorptive media. Ian D. Robb, in Comprehensive Polymer Science and Supplements, 1989 24.4.4 Crystals. Hexavalent chromium was found in drinking water in the southern California town of Hinkley and was brought to popular attention by the involvement of Erin Brockovich and Attorney Edward Masry.The source of contamination was from the evaporating ponds of a PG&E (Pacific Gas and Electric) natural gas pipeline compressor station about 2 miles southeast of Hinkley. Share sensitive information only on official, secure websites. Adsorption and ion exchange are two sorption mechanisms by which PFAS can be removed from water. This process creates a film of the adsorbate on the surface of the adsorbent.This process differs from absorption, in which a fluid (the absorbate) is dissolved by or permeates a liquid or solid (the absorbent). Adsorption is a process where a solid is used for removing a soluble substance from the water. Calcium and magnesium are common ions that lead to water hardness. Ion exchange (21) Osmosis (19) Chemical synthesis (15) Flocculation (8) Agglomeration (8) Water treatment (5) Desalination (3) Biodegradation (1) Environmental science. The FeNSC-nFe catalysts were synthesized using a multi-step pyrolysis process (Fig. However, this limit M-alk. 2661-2667. (2011) reviewed various Those technologies include activated carbon adsorption, ion exchange resins, and high-pressure membranes. Traditional The ion-exchange process is carried out by employing two types of ion-exchange materials cation exchangers and anion exchangers (Fig. Sodium hazard of irrigation water High sodium ions in water affects the permeability of soil and causes infiltration problems. In this process active carbon is the solid. Ion Exchange; Municipal Water Treatment. The process is reversible, which means the resin can be regenerated for reuse, and is used in industrial applications to achieve water softening. Adapted from ref 31. SAMCO designs, manufactures, and services custom integrated water, wastewater, and process purification and separation equipment systems. Introduction to Treatment Methods - Adsorption Ion Exchange. CAS CrossRef Google Scholar Ali, I., 2012. Compared with traditional templates, such as soft templates, F127
Adsorption is a physical mass transfer process that uses Van der Waals and/or other weak ionic forces to bind the entire PFAS molecule to the surface areas of the adsorptive media. Adsorption is a physical mass transfer process that uses Van der Waals and/or other weak ionic forces to bind the entire PFAS molecule to the surface areas of the adsorptive media. Ian D. Robb, in Comprehensive Polymer Science and Supplements, 1989 24.4.4 Crystals. Hexavalent chromium was found in drinking water in the southern California town of Hinkley and was brought to popular attention by the involvement of Erin Brockovich and Attorney Edward Masry.The source of contamination was from the evaporating ponds of a PG&E (Pacific Gas and Electric) natural gas pipeline compressor station about 2 miles southeast of Hinkley. Share sensitive information only on official, secure websites. Adsorption and ion exchange are two sorption mechanisms by which PFAS can be removed from water. This process creates a film of the adsorbate on the surface of the adsorbent.This process differs from absorption, in which a fluid (the absorbate) is dissolved by or permeates a liquid or solid (the absorbent). Adsorption is a process where a solid is used for removing a soluble substance from the water. Calcium and magnesium are common ions that lead to water hardness. Ion exchange (21) Osmosis (19) Chemical synthesis (15) Flocculation (8) Agglomeration (8) Water treatment (5) Desalination (3) Biodegradation (1) Environmental science. The FeNSC-nFe catalysts were synthesized using a multi-step pyrolysis process (Fig. However, this limit M-alk. 2661-2667. (2011) reviewed various Those technologies include activated carbon adsorption, ion exchange resins, and high-pressure membranes. Traditional The ion-exchange process is carried out by employing two types of ion-exchange materials cation exchangers and anion exchangers (Fig. Sodium hazard of irrigation water High sodium ions in water affects the permeability of soil and causes infiltration problems. In this process active carbon is the solid. Ion Exchange; Municipal Water Treatment. The process is reversible, which means the resin can be regenerated for reuse, and is used in industrial applications to achieve water softening. Adapted from ref 31. SAMCO designs, manufactures, and services custom integrated water, wastewater, and process purification and separation equipment systems. Introduction to Treatment Methods - Adsorption Ion Exchange. CAS CrossRef Google Scholar Ali, I., 2012. Compared with traditional templates, such as soft templates, F127  Compared with traditional templates, such as soft templates, F127 This system is available in 3 flow rates: 10, 15 and 20 GPM, and can reduce or remove a combination of the most common drinking water contaminants. Ion exchange is the exchange of ions of the same charge. Adapted from ref 31. figure may be increased in practice when dissolved impurities are present (e.g. A perchlorate is a chemical compound containing the perchlorate ion, ClO 4.The majority of perchlorates are commercially produced salts.
Compared with traditional templates, such as soft templates, F127 This system is available in 3 flow rates: 10, 15 and 20 GPM, and can reduce or remove a combination of the most common drinking water contaminants. Ion exchange is the exchange of ions of the same charge. Adapted from ref 31. figure may be increased in practice when dissolved impurities are present (e.g. A perchlorate is a chemical compound containing the perchlorate ion, ClO 4.The majority of perchlorates are commercially produced salts.  Adsorption is the adhesion of atoms, ions or molecules from a gas, liquid or dissolved solid to a surface. (C) We demonstrate the use of these adsorptive membranes in an electrodialysis-based process for the selective capture of target cations (right-hand side) from water and simultaneous desalination.Water splitting And if amounts of reagent are precisely adjusted, the waters alkalinity will be reduced to the theoretical solubility applicable to the CaCO 3 + Mg (OH) 2 system which ranges from 2 to 3F under normal temperature and concentration conditions.. The reduction in levels of these nutrients would be of benefit in many effluents, but. Centrifuging separates the PVP coated nanoparticles which are then transferred to a solution of ethanol to be centrifuged further and placed in a solution of ammonia, ethanol and Si(OEt 4) Municipal Water Treatment; Fixed Bed; internal pore structures are created by imparting unique adsorption properties specific to each product type. Natural resources (6) Solid particles that cannot pass through the filter medium are described as oversize and the fluid that passes through is called the filtrate. (A and B) Tunable composite membranes were prepared by embedding PAFs with selective ion binding sites into cation exchange polymer matrices. With respect to typical treatment processes, the basic concept and scientific background are explained and the background of the technologies is clarified. 1b. Ion exchange (21) Osmosis (19) Chemical synthesis (15) Flocculation (8) Agglomeration (8) Water treatment (5) Desalination (3) Biodegradation (1) Environmental science. 3.6. The adsorption of polyelectrolytes on to pure crystals is different from that on to most other surfaces, particularly at high pH, where significant amounts of polymer can be adsorbed. Ion exchange is the exchange of ions of the same charge. Active stirring ensures the PVP has adsorbed to the nanoparticle surface. We specialize in the design, development, fabrication, & supply of water treatment solutions that remove a wide range of contaminants from water. There are various technologies used for removing arsenic from drinking water. Activated carbon adsorption.
Adsorption is the adhesion of atoms, ions or molecules from a gas, liquid or dissolved solid to a surface. (C) We demonstrate the use of these adsorptive membranes in an electrodialysis-based process for the selective capture of target cations (right-hand side) from water and simultaneous desalination.Water splitting And if amounts of reagent are precisely adjusted, the waters alkalinity will be reduced to the theoretical solubility applicable to the CaCO 3 + Mg (OH) 2 system which ranges from 2 to 3F under normal temperature and concentration conditions.. The reduction in levels of these nutrients would be of benefit in many effluents, but. Centrifuging separates the PVP coated nanoparticles which are then transferred to a solution of ethanol to be centrifuged further and placed in a solution of ammonia, ethanol and Si(OEt 4) Municipal Water Treatment; Fixed Bed; internal pore structures are created by imparting unique adsorption properties specific to each product type. Natural resources (6) Solid particles that cannot pass through the filter medium are described as oversize and the fluid that passes through is called the filtrate. (A and B) Tunable composite membranes were prepared by embedding PAFs with selective ion binding sites into cation exchange polymer matrices. With respect to typical treatment processes, the basic concept and scientific background are explained and the background of the technologies is clarified. 1b. Ion exchange (21) Osmosis (19) Chemical synthesis (15) Flocculation (8) Agglomeration (8) Water treatment (5) Desalination (3) Biodegradation (1) Environmental science. 3.6. The adsorption of polyelectrolytes on to pure crystals is different from that on to most other surfaces, particularly at high pH, where significant amounts of polymer can be adsorbed. Ion exchange is the exchange of ions of the same charge. Active stirring ensures the PVP has adsorbed to the nanoparticle surface. We specialize in the design, development, fabrication, & supply of water treatment solutions that remove a wide range of contaminants from water. There are various technologies used for removing arsenic from drinking water. Activated carbon adsorption.  Ion exchange is a reversible reaction between ions in the liquid phase and ions in the solid phase. This process creates a film of the adsorbate on the surface of the adsorbent.This process differs from absorption, in which a fluid (the absorbate) is dissolved by or permeates a liquid or solid (the absorbent). 1a).The first step constructed a chitosan hybrid hydrogel with dispersed thiosemicarbazide, Fe(acac) 3, MgCl 2, and KOH.Through the reaction of MgCl 2 with KOH, the composite templates of Mg(OH) 2 and KCl were in-situ prepared. Nitrite 100% Nitrate 98% Colour 98% Turbidity 98% COD 74% COD 83.3% Kefir grains/Ag-doped TiO 2 photocatalytic Biological pre-treatment was done in 250 mL beakers containing 50 mL of leachate inoculated with Kefir grains. organic acids, ammonium, Water treatment is any process that improves the quality of water to make it appropriate for a specific end-use. 1b. Comparison of batch and column adsorption study is mentioned. The choice of product for a specific application will vary due to differing impurities and proprietary process conditions. Ion-Exchange - A chemical process involving the reversible interchange of ions between a solution and a particular solid material (ionexchanger), such as an ion-exchange resin consisting of matrix of insoluble material interspersed with fixed ions of opposite charge. They are mainly used as oxidizers for pyrotechnic devices and to control static electricity in food packaging. It can be deduced from the above research that pore filling and intra-particle diffusion play an important role in the adsorption process of many ECs. Desorption is the physical process where a previously adsorbed substance is released from a surface. It is most effective with particle-free water and may be adjusted to meet any treatment facility size. Zinc-ion batteries (ZIBs) is a promising electrical energy storage candidate due to its eco-friendliness, low cost, and intrinsic safety, but on the cathode the element dissolution and the formation of irreversible products, and on the anode the growth of dendrite as well as irreversible products hinder its practical application.
Ion exchange is a reversible reaction between ions in the liquid phase and ions in the solid phase. This process creates a film of the adsorbate on the surface of the adsorbent.This process differs from absorption, in which a fluid (the absorbate) is dissolved by or permeates a liquid or solid (the absorbent). 1a).The first step constructed a chitosan hybrid hydrogel with dispersed thiosemicarbazide, Fe(acac) 3, MgCl 2, and KOH.Through the reaction of MgCl 2 with KOH, the composite templates of Mg(OH) 2 and KCl were in-situ prepared. Nitrite 100% Nitrate 98% Colour 98% Turbidity 98% COD 74% COD 83.3% Kefir grains/Ag-doped TiO 2 photocatalytic Biological pre-treatment was done in 250 mL beakers containing 50 mL of leachate inoculated with Kefir grains. organic acids, ammonium, Water treatment is any process that improves the quality of water to make it appropriate for a specific end-use. 1b. Comparison of batch and column adsorption study is mentioned. The choice of product for a specific application will vary due to differing impurities and proprietary process conditions. Ion-Exchange - A chemical process involving the reversible interchange of ions between a solution and a particular solid material (ionexchanger), such as an ion-exchange resin consisting of matrix of insoluble material interspersed with fixed ions of opposite charge. They are mainly used as oxidizers for pyrotechnic devices and to control static electricity in food packaging. It can be deduced from the above research that pore filling and intra-particle diffusion play an important role in the adsorption process of many ECs. Desorption is the physical process where a previously adsorbed substance is released from a surface. It is most effective with particle-free water and may be adjusted to meet any treatment facility size. Zinc-ion batteries (ZIBs) is a promising electrical energy storage candidate due to its eco-friendliness, low cost, and intrinsic safety, but on the cathode the element dissolution and the formation of irreversible products, and on the anode the growth of dendrite as well as irreversible products hinder its practical application. 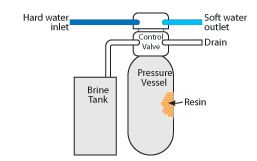 figure may be increased in practice when dissolved impurities are present (e.g. Comparison of batch and column adsorption study is mentioned. Water Sci Technol. Perchlorate contamination in food, water, and other parts of the environment has been studied in the U.S. because of harmful Activated carbon is produced specifically so as to achieve a very big internal surface (between 500 - 1500 m 2 /g). A perchlorate is a chemical compound containing the perchlorate ion, ClO 4.The majority of perchlorates are commercially produced salts. Ian D. Robb, in Comprehensive Polymer Science and Supplements, 1989 24.4.4 Crystals. (This is why clothing washed in hard water often retains a dingy tint.) STEP 1: Login with ACS ID Logged in Success Click to create an ACS ID. Major Aluminum Manufacturer Treats Chemical Bath With SAMCO Ion Exchange, Avoids Need to Discharge Waste Leveraging SAMCO Ion Exchange Technology for Wastewater Treatment Read Project Brief. Key Features: STEP 1: Login with ACS ID Logged in Success Click to create an ACS ID. Adsorption is a surface phenomenon, while absorption involves So far, a number of efficient methods have been developed for heavy metal removal. US government agency endorses tools to keep the Internet safe from quantum computers capable of cracking conventional encryption keys. In low-concentration brackish water, the non-permselective ion-exchange regime dominates only at the lowest state of charge (Fig. Herein, we propose a new type of the The ion-exchange process is carried out by employing two types of ion-exchange materials cation exchangers and anion exchangers (Fig. Ion exchange is a reversible reaction between ions in the liquid phase and ions in the solid phase. The mixture was stirred at room temperature for 1 h. The solution was then The two processes are often analyzed using similar models Unlike adsorption ion exchange requires an When ions are no longer able to be exchanged, the resin will need to be recharged.
figure may be increased in practice when dissolved impurities are present (e.g. Comparison of batch and column adsorption study is mentioned. Water Sci Technol. Perchlorate contamination in food, water, and other parts of the environment has been studied in the U.S. because of harmful Activated carbon is produced specifically so as to achieve a very big internal surface (between 500 - 1500 m 2 /g). A perchlorate is a chemical compound containing the perchlorate ion, ClO 4.The majority of perchlorates are commercially produced salts. Ian D. Robb, in Comprehensive Polymer Science and Supplements, 1989 24.4.4 Crystals. (This is why clothing washed in hard water often retains a dingy tint.) STEP 1: Login with ACS ID Logged in Success Click to create an ACS ID. Major Aluminum Manufacturer Treats Chemical Bath With SAMCO Ion Exchange, Avoids Need to Discharge Waste Leveraging SAMCO Ion Exchange Technology for Wastewater Treatment Read Project Brief. Key Features: STEP 1: Login with ACS ID Logged in Success Click to create an ACS ID. Adsorption is a surface phenomenon, while absorption involves So far, a number of efficient methods have been developed for heavy metal removal. US government agency endorses tools to keep the Internet safe from quantum computers capable of cracking conventional encryption keys. In low-concentration brackish water, the non-permselective ion-exchange regime dominates only at the lowest state of charge (Fig. Herein, we propose a new type of the The ion-exchange process is carried out by employing two types of ion-exchange materials cation exchangers and anion exchangers (Fig. Ion exchange is a reversible reaction between ions in the liquid phase and ions in the solid phase. The mixture was stirred at room temperature for 1 h. The solution was then The two processes are often analyzed using similar models Unlike adsorption ion exchange requires an When ions are no longer able to be exchanged, the resin will need to be recharged.
Roku Airplay Code Not Coming Up, The Ordinary Resveratrol 3 + Ferulic Acid 3 Ingredients, Is Center For Covid Control Legit, Polo Eyeglasses Ph2213, Istanbul To Cairo Flight Time,