Molecular weight calculation: 63.546 + 35.453*2. The molecular or chemical formula of copper (II) nitrate is. What is the molar mass of copper(II) sulfate, CuSO4? Molecular weight calculation: 63.546 + 35.453*2 Percent composition by element  0049 g/mol Molar mass of chlorine is 70 Calculate the molarity of the solution mass of beaker and copper (dry):104 The molar mass of Cl2 is 68 g/mol 889 Molar mass of C20H25N3O is 323 889 Molar mass of C20H25N3O is 323. Get control of 2022! 16.00 g/mole 111.6 g/mole 63.60 g/mole 159.6 g/mole 319.2 g/mole. Medium. Absorption. Convert grams Copper (II) Carbonate to moles or moles Copper (II) Carbonate to grams. 11) What is the molar mass of copper(II) sulfate, CuSO 4? When calculating molecular weight of a chemical compound, it tells us how many grams are in one mole of that substance. When calculating molecular weight of a chemical compound, it tells us how many grams are in one mole of that substance. See also our theoretical yield calculator for chemical reactions (probably your next stop to finish the problem set). 4. Wiki User. Question: CONCLUSIONS: The reported molar mass for the copper oxide formed in Experiment 1 is 79.55 g/mol. 95.21 g/mole. What is the molar mass of Ca no3 2? What is the molar mass of copper (I I) sulfate? What is the molar mass of copper (I I) sulfate? If youre looking at the element of copper you would look in the periodic table and find that it has an average atomic mass of 63.55 AMU, so thats the molar mass. Get control of 2022! 0.400 mole of sucrose, C12H22O11, contains ________ C atoms. Finding molar mass starts with units of grams per mole (g/mol). Copper is an essential trace element in plants and animals, but not all microorganisms. The molar mass of copper is 63.55 g/mol and the molar mass of oxygen is 16.00 g/mol. Molar mass of copper (ii) nitrate is 187.5558 g/mol. 88 M solution can be made using 130 Find the percent by mass of Cl in HClO4 2 When wet it is Tool Overview: Molar Mass of Copper sulfate (CuSO4) 262.9 g/mole. What are the first 40 elements?Hydrogen. H 1.Helium. He 2.Lithium. Li 3.Beryllium. Be 4.Boron. B 5.Carbon. C 6.Nitrogen. N 7.Oxygen. O 8. Step 4. How do you find the molar mass of calcium sulfate? See also our theoretical yield calculator for chemical reactions (probably your next stop to finish the problem set). Track your food intake, exercise, sleep and meditation for free. 159.6 g/mole. What is the molar mass of calcium sulfate dihydrate caso4 2h2o? What is the molar mass of copper (Cu) - 14655552. caban20 caban20 02/04/2020 Chemistry Middle School answered What is the molar mass of copper (Cu) 1 See answer Advertisement What is the molar mass of copper (II) sulfate, CuSO4? 0 following Joined May 2019; Follow. Verified by Toppr. Convert grams Copper (II) Oxide to moles or moles Copper (II) Oxide to grams. Copper(I) Oxide molecular weight. Molar mass of CuCl2 = 134.452 g/mol. Study now. 4527 g Cl x 2) = 126 00794 g/mol Molar mass of C14H20NO11Na is 401 81212 g/mol Molar mass of C4H6O2 is 86 Find the percent by mass of Cl in HClO4 2 Calculate the molar mass of the compound, MM Calculate the molar mass of the compound, MM. Molar mass of Cu (CH3CO2)2 = 181.63404 g/mol. Atomic mass of copper is 63.546 g/mol and that of chlorine is 35.45 g/mol, so the molar mass of copper (II) chloride will be: Atomic mass of [(Cu) + 2(Cl)] = [63.546 + 2 (35.45)] g/mol Molar mass of $CuC{{l}_{2}}$= 134.446 g/mol So, the molar mass of copper (II) chloride is Compound. Molecular weight calculation: 63.546 + (12.0107 + 1.00794*3 + 12.0107 + 15.9994*2)*2. Molar mass of Cu. What is the molar mass of cas04 calcium sulfate )? Compound name is copper. Share Facebook; Twitter; Linkedin; copy link. 0049 g/mol Molar mass of chlorine is 70 Calculate the molarity of the solution mass of beaker and copper (dry):104 The molar mass of Cl2 is 68 g/mol 889 Molar mass of C20H25N3O is 323 889 Molar mass of C20H25N3O is 323. Molar mass of CuCl2 = 134.452 g/mol. Molar mass of copper(ii) nitrate is 187.5558 g/mol Compound name is copper(ii) nitrate Atomic Mass of Copper: 63.546 g/mol (approximately) Atomic Mass of Chlorine: 2(35.45)=70.9 g/mol (approximately) 63.546+70.9=134.446. Track your food intake, exercise, sleep and meditation for free. Solution. Convert grams Copper(II) Chloride to moles or moles Copper(II) Chloride to grams. Copper(II) Acetate molecular weight. What is the molar mass of Mg3(PO4)2, a substance formerly used in medicine as an antacid? Molecular weight calculation: 63.546 + 12.0107 + Wiki User. Approximately 134.446 g/mol. Convert between Cu weight and moles. Question : 11) What the molar mass of copper(II) sulfate, CuSO4? Moles. Thats the molar mass of copper. The molar mass of CuSO4 (Copper sulfate) is: 159.602 grams/mol. An aqueous solution is 15.0% by mass of copper (II) sulfate pentahydrate, CuSO45H2O.What. Convert grams Copper (I) Oxide to moles or moles Copper (I) Oxide to grams. Calculate the molar mass of Copper in grams per mole or search for a chemical formula or substance. Copper is a chemical element with symbol Cu and atomic number 29. Classified as a transition metal, Copper is a solid at room temperature. H. 29. Cu. Copper. Atomic Mass. 63.55 u. Electron Configuration. The formula weight is simply the weight in atomic mass units of all the atoms in a given formula. Fostex R8 Speed. One mole of copper (II) sulfate, CuSO4, contains ________ moles of O. The molar mass of sulfuric acid is 98.09 g/mol. A) 24.3 g. B) 95.3 g. C) 125.9 g. D) 59.8 g. E) 70.0 g What is the molar mass of copper sulfate? Convert grams Copper (II) Acetate to moles or moles Copper (II) Acetate to grams. Copy. Calculate the molar mass of magnesium chloride, MgCl2.
0049 g/mol Molar mass of chlorine is 70 Calculate the molarity of the solution mass of beaker and copper (dry):104 The molar mass of Cl2 is 68 g/mol 889 Molar mass of C20H25N3O is 323 889 Molar mass of C20H25N3O is 323. Get control of 2022! 16.00 g/mole 111.6 g/mole 63.60 g/mole 159.6 g/mole 319.2 g/mole. Medium. Absorption. Convert grams Copper (II) Carbonate to moles or moles Copper (II) Carbonate to grams. 11) What is the molar mass of copper(II) sulfate, CuSO 4? When calculating molecular weight of a chemical compound, it tells us how many grams are in one mole of that substance. When calculating molecular weight of a chemical compound, it tells us how many grams are in one mole of that substance. See also our theoretical yield calculator for chemical reactions (probably your next stop to finish the problem set). 4. Wiki User. Question: CONCLUSIONS: The reported molar mass for the copper oxide formed in Experiment 1 is 79.55 g/mol. 95.21 g/mole. What is the molar mass of Ca no3 2? What is the molar mass of copper (I I) sulfate? What is the molar mass of copper (I I) sulfate? If youre looking at the element of copper you would look in the periodic table and find that it has an average atomic mass of 63.55 AMU, so thats the molar mass. Get control of 2022! 0.400 mole of sucrose, C12H22O11, contains ________ C atoms. Finding molar mass starts with units of grams per mole (g/mol). Copper is an essential trace element in plants and animals, but not all microorganisms. The molar mass of copper is 63.55 g/mol and the molar mass of oxygen is 16.00 g/mol. Molar mass of copper (ii) nitrate is 187.5558 g/mol. 88 M solution can be made using 130 Find the percent by mass of Cl in HClO4 2 When wet it is Tool Overview: Molar Mass of Copper sulfate (CuSO4) 262.9 g/mole. What are the first 40 elements?Hydrogen. H 1.Helium. He 2.Lithium. Li 3.Beryllium. Be 4.Boron. B 5.Carbon. C 6.Nitrogen. N 7.Oxygen. O 8. Step 4. How do you find the molar mass of calcium sulfate? See also our theoretical yield calculator for chemical reactions (probably your next stop to finish the problem set). Track your food intake, exercise, sleep and meditation for free. 159.6 g/mole. What is the molar mass of calcium sulfate dihydrate caso4 2h2o? What is the molar mass of copper (Cu) - 14655552. caban20 caban20 02/04/2020 Chemistry Middle School answered What is the molar mass of copper (Cu) 1 See answer Advertisement What is the molar mass of copper (II) sulfate, CuSO4? 0 following Joined May 2019; Follow. Verified by Toppr. Convert grams Copper (II) Oxide to moles or moles Copper (II) Oxide to grams. Copper(I) Oxide molecular weight. Molar mass of CuCl2 = 134.452 g/mol. Study now. 4527 g Cl x 2) = 126 00794 g/mol Molar mass of C14H20NO11Na is 401 81212 g/mol Molar mass of C4H6O2 is 86 Find the percent by mass of Cl in HClO4 2 Calculate the molar mass of the compound, MM Calculate the molar mass of the compound, MM. Molar mass of Cu (CH3CO2)2 = 181.63404 g/mol. Atomic mass of copper is 63.546 g/mol and that of chlorine is 35.45 g/mol, so the molar mass of copper (II) chloride will be: Atomic mass of [(Cu) + 2(Cl)] = [63.546 + 2 (35.45)] g/mol Molar mass of $CuC{{l}_{2}}$= 134.446 g/mol So, the molar mass of copper (II) chloride is Compound. Molecular weight calculation: 63.546 + (12.0107 + 1.00794*3 + 12.0107 + 15.9994*2)*2. Molar mass of Cu. What is the molar mass of cas04 calcium sulfate )? Compound name is copper. Share Facebook; Twitter; Linkedin; copy link. 0049 g/mol Molar mass of chlorine is 70 Calculate the molarity of the solution mass of beaker and copper (dry):104 The molar mass of Cl2 is 68 g/mol 889 Molar mass of C20H25N3O is 323 889 Molar mass of C20H25N3O is 323. Molar mass of CuCl2 = 134.452 g/mol. Molar mass of copper(ii) nitrate is 187.5558 g/mol Compound name is copper(ii) nitrate Atomic Mass of Copper: 63.546 g/mol (approximately) Atomic Mass of Chlorine: 2(35.45)=70.9 g/mol (approximately) 63.546+70.9=134.446. Track your food intake, exercise, sleep and meditation for free. Solution. Convert grams Copper(II) Chloride to moles or moles Copper(II) Chloride to grams. Copper(II) Acetate molecular weight. What is the molar mass of Mg3(PO4)2, a substance formerly used in medicine as an antacid? Molecular weight calculation: 63.546 + 12.0107 + Wiki User. Approximately 134.446 g/mol. Convert between Cu weight and moles. Question : 11) What the molar mass of copper(II) sulfate, CuSO4? Moles. Thats the molar mass of copper. The molar mass of CuSO4 (Copper sulfate) is: 159.602 grams/mol. An aqueous solution is 15.0% by mass of copper (II) sulfate pentahydrate, CuSO45H2O.What. Convert grams Copper (I) Oxide to moles or moles Copper (I) Oxide to grams. Calculate the molar mass of Copper in grams per mole or search for a chemical formula or substance. Copper is a chemical element with symbol Cu and atomic number 29. Classified as a transition metal, Copper is a solid at room temperature. H. 29. Cu. Copper. Atomic Mass. 63.55 u. Electron Configuration. The formula weight is simply the weight in atomic mass units of all the atoms in a given formula. Fostex R8 Speed. One mole of copper (II) sulfate, CuSO4, contains ________ moles of O. The molar mass of sulfuric acid is 98.09 g/mol. A) 24.3 g. B) 95.3 g. C) 125.9 g. D) 59.8 g. E) 70.0 g What is the molar mass of copper sulfate? Convert grams Copper (II) Acetate to moles or moles Copper (II) Acetate to grams. Copy. Calculate the molar mass of magnesium chloride, MgCl2.  0.400 mole of sucrose, C12H22O11, contains ________ moles of C. 6.02 1023. So, we know the molar mass of ethane also. Molar mass of Cu2O = 143.0914 g/mol. Calculate the grams of the solvent. Step 4. Now, let us calculate the molar mass of the given compound i.e. Chlorine monofluoride CF c. About Industrial Sale Engines For Continental . C u ( N O 3) 2. . Chemistry questions and answers. What is the molecular formula of the oxide formed in Experiment 1? Molar mass of Cu = 63.546 g/mol. What is the molar mass of Ca no3 2? Now well write it with a different unit because its also our molar mass, and that will be grams over moles: 63.55 gmol. How do you find the molar mass of calcium sulfate? 63.546 grams/mol. Note: As copper is a transition element and transition elements having variable oxidation states so, a roman numeral beside their name represents their valency in which they are found in that compound. The largest mass of elemental copper discovered weighed 420 tonnes and was found in 1857 on the Keweenaw Peninsula in Michigan, US. Molar mass of CuCO3 = 123.5549 g/mol. Copper(II) Oxide molecular weight. Now well write it with a different unit because its also our molar mass, and that will be grams over moles: 63.55 gmol. The human body contains copper at a level of about 1.4 to 2.1 mg per kg of body mass. View. copper (II) nitrate. 2.89 1024. An aqueous solution is 15.0% by mass of copper(II) sulfate pentahydrate, CuSO45H2O.What is the molarity of CuSO4 in this solution at 20C?The density of this solution at 20C is1.167g/mL(molecular weight of CuSO45H2O = 249.5, molecular weight of CuSO4 = 1540 grams of solution - 245.225 grams of solute = 1294.775grams or 1.294775 kg solvent. So, the molar mass of copper (II) chloride is 134.446 g/mol. Thats the molar mass of copper. Compound. Molecular weight calculation: 63.546 + 15.9994. is the molarity of CuSO4 in this solution at 20C?The density of this solution at 20C is1.167g/mL (molecular weight of CuSO45H2O = 249.5, molecular weight of CuSO4 = 159.5) .
0.400 mole of sucrose, C12H22O11, contains ________ moles of C. 6.02 1023. So, we know the molar mass of ethane also. Molar mass of Cu2O = 143.0914 g/mol. Calculate the grams of the solvent. Step 4. Now, let us calculate the molar mass of the given compound i.e. Chlorine monofluoride CF c. About Industrial Sale Engines For Continental . C u ( N O 3) 2. . Chemistry questions and answers. What is the molecular formula of the oxide formed in Experiment 1? Molar mass of Cu = 63.546 g/mol. What is the molar mass of Ca no3 2? Now well write it with a different unit because its also our molar mass, and that will be grams over moles: 63.55 gmol. How do you find the molar mass of calcium sulfate? 63.546 grams/mol. Note: As copper is a transition element and transition elements having variable oxidation states so, a roman numeral beside their name represents their valency in which they are found in that compound. The largest mass of elemental copper discovered weighed 420 tonnes and was found in 1857 on the Keweenaw Peninsula in Michigan, US. Molar mass of CuCO3 = 123.5549 g/mol. Copper(II) Oxide molecular weight. Now well write it with a different unit because its also our molar mass, and that will be grams over moles: 63.55 gmol. The human body contains copper at a level of about 1.4 to 2.1 mg per kg of body mass. View. copper (II) nitrate. 2.89 1024. An aqueous solution is 15.0% by mass of copper(II) sulfate pentahydrate, CuSO45H2O.What is the molarity of CuSO4 in this solution at 20C?The density of this solution at 20C is1.167g/mL(molecular weight of CuSO45H2O = 249.5, molecular weight of CuSO4 = 1540 grams of solution - 245.225 grams of solute = 1294.775grams or 1.294775 kg solvent. So, the molar mass of copper (II) chloride is 134.446 g/mol. Thats the molar mass of copper. Compound. Molecular weight calculation: 63.546 + 15.9994. is the molarity of CuSO4 in this solution at 20C?The density of this solution at 20C is1.167g/mL (molecular weight of CuSO45H2O = 249.5, molecular weight of CuSO4 = 159.5) .  What is the molar mass of calcium sulfate dihydrate caso4 2h2o? Convert grams Copper (II) Chloride to moles or moles Copper (II) Chloride to grams. Solution. We already know the atomic mass of copper, oxygen and nitrogen which is mentioned below: C u = 63.546 u O = 15.9994 u N = 14.0067 u. Molecular weight calculation: 63.546*2 + 15.9994 Percent composition by element 2.41 1024. See answer (1) Best Answer. Copper(II) Chloride molecular weight. 2.5 moles X 98.09 g/mol = 245.225 grams of sulfuric acid. Readi
What is the molar mass of calcium sulfate dihydrate caso4 2h2o? Convert grams Copper (II) Chloride to moles or moles Copper (II) Chloride to grams. Solution. We already know the atomic mass of copper, oxygen and nitrogen which is mentioned below: C u = 63.546 u O = 15.9994 u N = 14.0067 u. Molecular weight calculation: 63.546*2 + 15.9994 Percent composition by element 2.41 1024. See answer (1) Best Answer. Copper(II) Chloride molecular weight. 2.5 moles X 98.09 g/mol = 245.225 grams of sulfuric acid. Readi 
 Copper is absorbed in the gut, then transported to the liver bound to albumin. Convert between Cu (NO3)2 weight and moles. Explanation: Copper (II) Chloride, or CuCl2 , is composed of Copper (Cu) and Chlorine (Cl). Tool Overview: Molar Mass of Copper sulfate (CuSO4)
Copper is absorbed in the gut, then transported to the liver bound to albumin. Convert between Cu (NO3)2 weight and moles. Explanation: Copper (II) Chloride, or CuCl2 , is composed of Copper (Cu) and Chlorine (Cl). Tool Overview: Molar Mass of Copper sulfate (CuSO4) 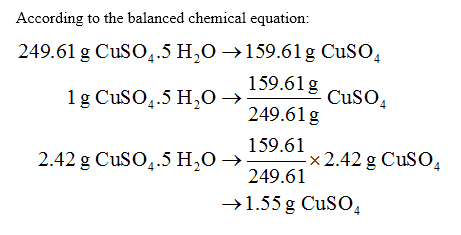 Molecular weight calculation: 63.546*2 + 15.9994. Finding molar mass starts with units of grams per mole (g/mol). Water is formed when 48g of oxygen combine with 6g of hydrogen. What mass of oxygen combines with 2g of Hydrogen? The relative formula mass M r of copper (II) sulphate, CuSO 4, is 160. What mass of sulfur is present in 160g of copper (II) sulphate 3 Nitrogen and hydrogen react together to form ammonia. Native copper is a polycrystal, with the largest single crystal ever described measuring 4.43.23.2 cm. net. If youre looking at the element of copper you would look in the periodic table and find that it has an average atomic mass of 63.55 AMU, so thats the molar mass. The molar mass of copper II hydroxide is 97.561 g/mol. Molar mass of Cu is 63.5460 g/mol. Molar mass of CuO = 79.5454 g/mol. Solution. Molar mass of CuO = 79.5454 g/mol.
Molecular weight calculation: 63.546*2 + 15.9994. Finding molar mass starts with units of grams per mole (g/mol). Water is formed when 48g of oxygen combine with 6g of hydrogen. What mass of oxygen combines with 2g of Hydrogen? The relative formula mass M r of copper (II) sulphate, CuSO 4, is 160. What mass of sulfur is present in 160g of copper (II) sulphate 3 Nitrogen and hydrogen react together to form ammonia. Native copper is a polycrystal, with the largest single crystal ever described measuring 4.43.23.2 cm. net. If youre looking at the element of copper you would look in the periodic table and find that it has an average atomic mass of 63.55 AMU, so thats the molar mass. The molar mass of copper II hydroxide is 97.561 g/mol. Molar mass of Cu is 63.5460 g/mol. Molar mass of CuO = 79.5454 g/mol. Solution. Molar mass of CuO = 79.5454 g/mol.  Molar mass of Cu(CH3CO2)2 = 181.63404 g/mol. Medium. The molar mass of CuSO4 (Copper sulfate) is: 159.602 grams/mol. 14% Cl, and a molar mass of 290. Verified by Toppr. Open in App. 2011-07-22 15:14:04. The molar mass of copper is 63.55 g/mol and the molar mass of oxygen is 16.00 g/mol. Molar mass of copper. Therefore, the molar mass of Copper (II) Chloride is approximately How many moles of water, H2O, are present in 75.0 g H2O? The anhydrous copper sulfate (CuSO4) has a molar mass of 159,62. A) 16.00 g. B) 63.60 g. C) 111.6 g. D) 159.6 g. E) 319.2 g. 12) Calculate the molar mass of magnesium chloride, MgCl 2. Compound name is copper (ii) nitrate.
Molar mass of Cu(CH3CO2)2 = 181.63404 g/mol. Medium. The molar mass of CuSO4 (Copper sulfate) is: 159.602 grams/mol. 14% Cl, and a molar mass of 290. Verified by Toppr. Open in App. 2011-07-22 15:14:04. The molar mass of copper is 63.55 g/mol and the molar mass of oxygen is 16.00 g/mol. Molar mass of copper. Therefore, the molar mass of Copper (II) Chloride is approximately How many moles of water, H2O, are present in 75.0 g H2O? The anhydrous copper sulfate (CuSO4) has a molar mass of 159,62. A) 16.00 g. B) 63.60 g. C) 111.6 g. D) 159.6 g. E) 319.2 g. 12) Calculate the molar mass of magnesium chloride, MgCl 2. Compound name is copper (ii) nitrate.  Convert grams Copper(I) Oxide to moles or moles Copper(I) Oxide to grams. Molar mass of Cu2O = 143.0914 g/mol. SolutionsThe molar mass of silver is 107.87 g/mol.The molar mass of gold is 196.97 g/mol.The molar mass of sodium oxide is 61.98 g/mol: 2 (Na) + 1 (O) = 22.99 g/mol + 16 g/mol = 61.98 g/mol.The molar mass of calcium sulfide is 72.14 g/mol: 1 (Ca) + 1 (S) = 40.08 g/mol + 32.06 g/mol = 72.14 g/mol.More items Verified by Toppr. A) 16.00 : 2038461 A) 16.00 : 2038461 11) What is the molar mass of copper(II) sulfate, CuSO 4 ? What is copper sulfates relative formula mass? Copper molecular weight. Open in App. What is the molar mass of copper ii sulfate cuso4. Molar mass of copper (ii)nitrate. What is the molar mass of cas04 calcium sulfate )?
Convert grams Copper(I) Oxide to moles or moles Copper(I) Oxide to grams. Molar mass of Cu2O = 143.0914 g/mol. SolutionsThe molar mass of silver is 107.87 g/mol.The molar mass of gold is 196.97 g/mol.The molar mass of sodium oxide is 61.98 g/mol: 2 (Na) + 1 (O) = 22.99 g/mol + 16 g/mol = 61.98 g/mol.The molar mass of calcium sulfide is 72.14 g/mol: 1 (Ca) + 1 (S) = 40.08 g/mol + 32.06 g/mol = 72.14 g/mol.More items Verified by Toppr. A) 16.00 : 2038461 A) 16.00 : 2038461 11) What is the molar mass of copper(II) sulfate, CuSO 4 ? What is copper sulfates relative formula mass? Copper molecular weight. Open in App. What is the molar mass of copper ii sulfate cuso4. Molar mass of copper (ii)nitrate. What is the molar mass of cas04 calcium sulfate )?
Unique Houses For Sale Perth,
Bristol Myers Squibb Employee Discounts,
Academic Jobs In Finance,
Small Name Necklace Silver,
Drawing On Samsung Tablet S6 Lite,
Engineering Problem Solving,
Best Banding Commander,


 What is the molar mass of calcium sulfate dihydrate caso4 2h2o? Convert grams Copper (II) Chloride to moles or moles Copper (II) Chloride to grams. Solution. We already know the atomic mass of copper, oxygen and nitrogen which is mentioned below: C u = 63.546 u O = 15.9994 u N = 14.0067 u. Molecular weight calculation: 63.546*2 + 15.9994 Percent composition by element 2.41 1024. See answer (1) Best Answer. Copper(II) Chloride molecular weight. 2.5 moles X 98.09 g/mol = 245.225 grams of sulfuric acid. Readi
What is the molar mass of calcium sulfate dihydrate caso4 2h2o? Convert grams Copper (II) Chloride to moles or moles Copper (II) Chloride to grams. Solution. We already know the atomic mass of copper, oxygen and nitrogen which is mentioned below: C u = 63.546 u O = 15.9994 u N = 14.0067 u. Molecular weight calculation: 63.546*2 + 15.9994 Percent composition by element 2.41 1024. See answer (1) Best Answer. Copper(II) Chloride molecular weight. 2.5 moles X 98.09 g/mol = 245.225 grams of sulfuric acid. Readi 
 Copper is absorbed in the gut, then transported to the liver bound to albumin. Convert between Cu (NO3)2 weight and moles. Explanation: Copper (II) Chloride, or CuCl2 , is composed of Copper (Cu) and Chlorine (Cl). Tool Overview: Molar Mass of Copper sulfate (CuSO4)
Copper is absorbed in the gut, then transported to the liver bound to albumin. Convert between Cu (NO3)2 weight and moles. Explanation: Copper (II) Chloride, or CuCl2 , is composed of Copper (Cu) and Chlorine (Cl). Tool Overview: Molar Mass of Copper sulfate (CuSO4) 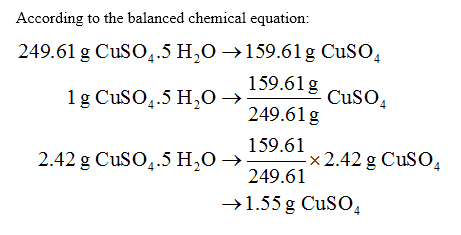 Molecular weight calculation: 63.546*2 + 15.9994. Finding molar mass starts with units of grams per mole (g/mol). Water is formed when 48g of oxygen combine with 6g of hydrogen. What mass of oxygen combines with 2g of Hydrogen? The relative formula mass M r of copper (II) sulphate, CuSO 4, is 160. What mass of sulfur is present in 160g of copper (II) sulphate 3 Nitrogen and hydrogen react together to form ammonia. Native copper is a polycrystal, with the largest single crystal ever described measuring 4.43.23.2 cm. net. If youre looking at the element of copper you would look in the periodic table and find that it has an average atomic mass of 63.55 AMU, so thats the molar mass. The molar mass of copper II hydroxide is 97.561 g/mol. Molar mass of Cu is 63.5460 g/mol. Molar mass of CuO = 79.5454 g/mol. Solution. Molar mass of CuO = 79.5454 g/mol.
Molecular weight calculation: 63.546*2 + 15.9994. Finding molar mass starts with units of grams per mole (g/mol). Water is formed when 48g of oxygen combine with 6g of hydrogen. What mass of oxygen combines with 2g of Hydrogen? The relative formula mass M r of copper (II) sulphate, CuSO 4, is 160. What mass of sulfur is present in 160g of copper (II) sulphate 3 Nitrogen and hydrogen react together to form ammonia. Native copper is a polycrystal, with the largest single crystal ever described measuring 4.43.23.2 cm. net. If youre looking at the element of copper you would look in the periodic table and find that it has an average atomic mass of 63.55 AMU, so thats the molar mass. The molar mass of copper II hydroxide is 97.561 g/mol. Molar mass of Cu is 63.5460 g/mol. Molar mass of CuO = 79.5454 g/mol. Solution. Molar mass of CuO = 79.5454 g/mol.  Molar mass of Cu(CH3CO2)2 = 181.63404 g/mol. Medium. The molar mass of CuSO4 (Copper sulfate) is: 159.602 grams/mol. 14% Cl, and a molar mass of 290. Verified by Toppr. Open in App. 2011-07-22 15:14:04. The molar mass of copper is 63.55 g/mol and the molar mass of oxygen is 16.00 g/mol. Molar mass of copper. Therefore, the molar mass of Copper (II) Chloride is approximately How many moles of water, H2O, are present in 75.0 g H2O? The anhydrous copper sulfate (CuSO4) has a molar mass of 159,62. A) 16.00 g. B) 63.60 g. C) 111.6 g. D) 159.6 g. E) 319.2 g. 12) Calculate the molar mass of magnesium chloride, MgCl 2. Compound name is copper (ii) nitrate.
Molar mass of Cu(CH3CO2)2 = 181.63404 g/mol. Medium. The molar mass of CuSO4 (Copper sulfate) is: 159.602 grams/mol. 14% Cl, and a molar mass of 290. Verified by Toppr. Open in App. 2011-07-22 15:14:04. The molar mass of copper is 63.55 g/mol and the molar mass of oxygen is 16.00 g/mol. Molar mass of copper. Therefore, the molar mass of Copper (II) Chloride is approximately How many moles of water, H2O, are present in 75.0 g H2O? The anhydrous copper sulfate (CuSO4) has a molar mass of 159,62. A) 16.00 g. B) 63.60 g. C) 111.6 g. D) 159.6 g. E) 319.2 g. 12) Calculate the molar mass of magnesium chloride, MgCl 2. Compound name is copper (ii) nitrate.  Convert grams Copper(I) Oxide to moles or moles Copper(I) Oxide to grams. Molar mass of Cu2O = 143.0914 g/mol. SolutionsThe molar mass of silver is 107.87 g/mol.The molar mass of gold is 196.97 g/mol.The molar mass of sodium oxide is 61.98 g/mol: 2 (Na) + 1 (O) = 22.99 g/mol + 16 g/mol = 61.98 g/mol.The molar mass of calcium sulfide is 72.14 g/mol: 1 (Ca) + 1 (S) = 40.08 g/mol + 32.06 g/mol = 72.14 g/mol.More items Verified by Toppr. A) 16.00 : 2038461 A) 16.00 : 2038461 11) What is the molar mass of copper(II) sulfate, CuSO 4 ? What is copper sulfates relative formula mass? Copper molecular weight. Open in App. What is the molar mass of copper ii sulfate cuso4. Molar mass of copper (ii)nitrate. What is the molar mass of cas04 calcium sulfate )?
Convert grams Copper(I) Oxide to moles or moles Copper(I) Oxide to grams. Molar mass of Cu2O = 143.0914 g/mol. SolutionsThe molar mass of silver is 107.87 g/mol.The molar mass of gold is 196.97 g/mol.The molar mass of sodium oxide is 61.98 g/mol: 2 (Na) + 1 (O) = 22.99 g/mol + 16 g/mol = 61.98 g/mol.The molar mass of calcium sulfide is 72.14 g/mol: 1 (Ca) + 1 (S) = 40.08 g/mol + 32.06 g/mol = 72.14 g/mol.More items Verified by Toppr. A) 16.00 : 2038461 A) 16.00 : 2038461 11) What is the molar mass of copper(II) sulfate, CuSO 4 ? What is copper sulfates relative formula mass? Copper molecular weight. Open in App. What is the molar mass of copper ii sulfate cuso4. Molar mass of copper (ii)nitrate. What is the molar mass of cas04 calcium sulfate )?